Single Site Catalysis
For dehydrogenation of formic acid:
Metal–organic frameworks (MOFs) are ideal hosts for incorporation of molecular complexes without altering their original ligand environment; molecular catalysts can thus be easily synthesized and used in gas- and vapor-phase reactions operated in continuous mode.
We report the immobilization of a molecular ruthenium complex in a phosphine-functionalized MOF that is highly efficient in the vapor-phase dehydrogenation of formic acid.
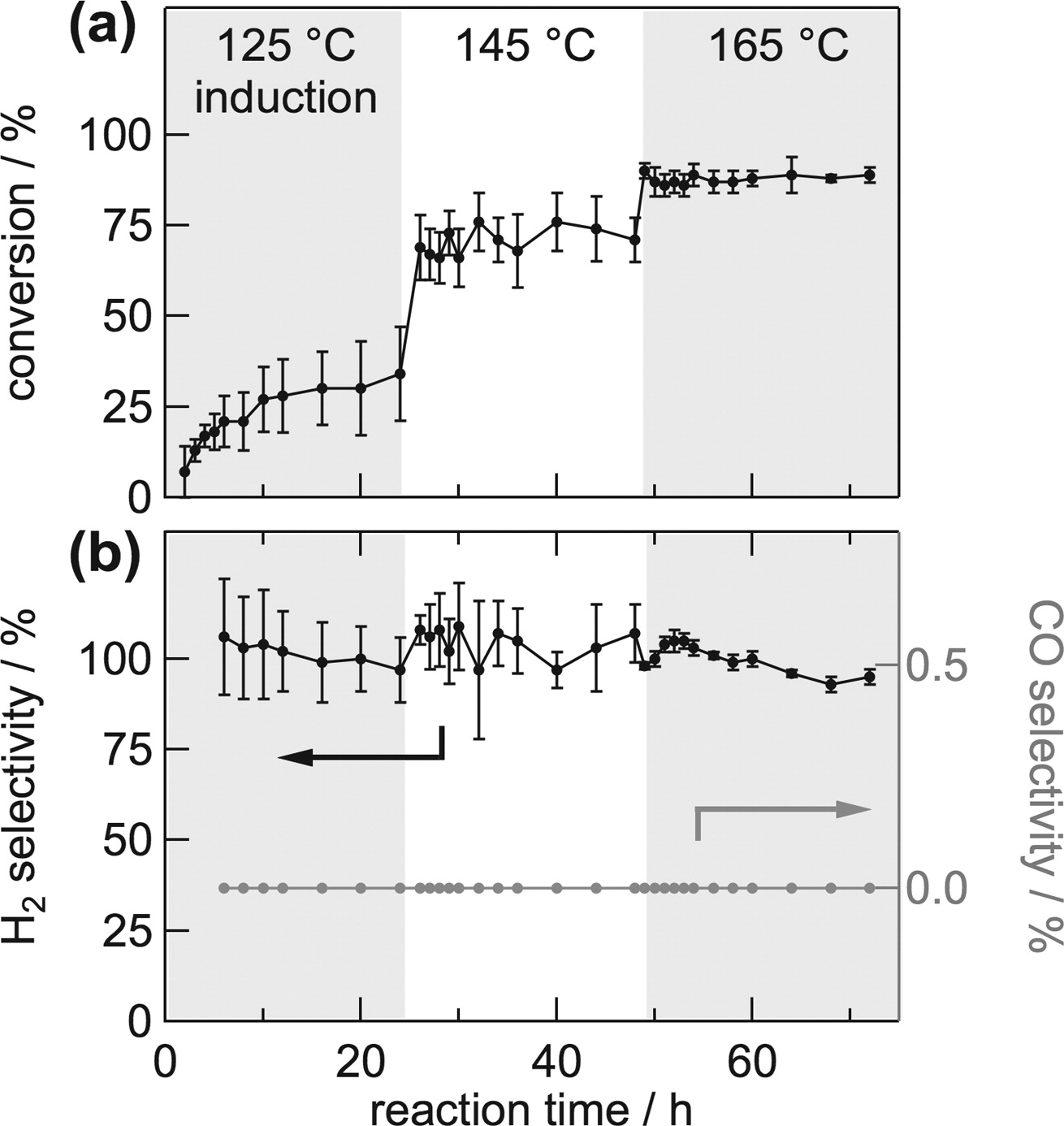
The catalyst exhibited exclusive selectivity to hydrogen and carbon dioxide with outstanding stability at 145 °C (TON > 1 290 000).
Our results represent a noteworthy improvement over heterogeneous ruthenium systems in terms of selectivity in the gas-phase, while reaching a productivity level higher than that of state-of-the-art homogeneous catalysts.
This research is published by: A. B. Redondo, F. L. Morel, M. Ranocchiari, and J. A. van Bokhoven, Functionalized Ruthenium–Phosphine Metal–Organic Framework for Continuous Vapor-Phase Dehydrogenation of Formic Acid, ACS Catal., 2015, 5 (12), pp 7099–7103
external pageDOI:10.1021/acscatal.5b01987call_made