Hydrogen Production
Nanoparticles of metal-oxide semiconductors such as titania (TiO2) or zinc oxide (ZnO) are known to be active photocatalysts. One important example is solar water splitting over titania for the generation of hydrogen gas that serves as an environmentally friendly fuel. Recently, it has been shown that adding noble metal nanoparticles may significantly enhance the catalytic activity.
With the aim of optimizing the photocatalysts for the envisaged processes, we study their dynamics in time-resolved experiments. Here, a short laser pulse (called pump) takes the role of the sun and photoexcites electrons and holes. Their evolution is then monitored using a second (probe) pulse, which may be optical from a laser or x-rays from a synchrotron. Varying the time delay between pump and probe reveals the dynamics of interest.
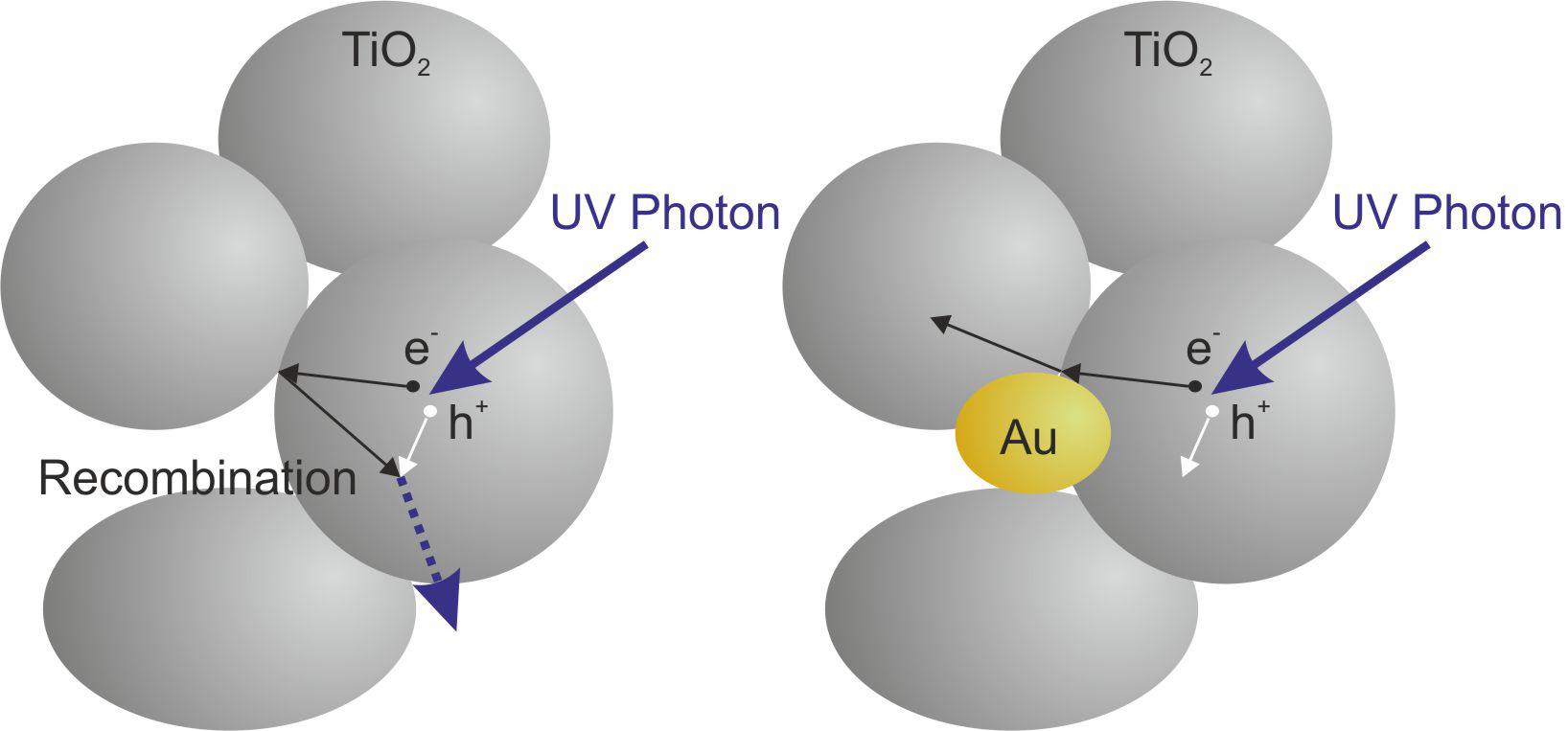
In such a pump/probe measurement, we observed a threefold increase in the absorption of a far-infrared probe pulse in the presence of gold nanoparticles compared to pure titania after illumination with an ultraviolet pump pulse. This is an indication that the gold leads to a larger number of photoexcited free electrons. We attribute this to an efficient separation of the electrons and holes in the vicinity of the gold particles (see figure), which in turn leads to a reduced recombination rate.
The availability of more conduction band electrons is, at least partially, responsible for the enhanced activity of gold-on-titania observed in catalytic experiments.
